electronic configuration of cr3|Chromium : Tagatay Since 1s can only hold two electrons the next 2 electrons for magnesium go in . SOON_ is a digital product agency providing high-quality, bespoke web-based solutions that revolutionise businesses. . We're in the business of change. We’re an award-winning digital product and eCommerce agency providing high-quality, bespoke web-based solutions that revolutionise businesses. Go to homepage. eCommerce. . London SE1 .
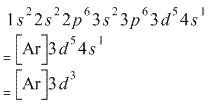
electronic configuration of cr3,How to Write the Electron Configuration for Chromium (Cr, Cr2+, and Cr3+) In order to write the Chromium electron configuration we first need to know the number of electrons for the Cr atom (there are 24 electrons). Once we have the configuration for Cr, the ions are .
electronic configuration of cr3Both of the configurations have the correct numbers of electrons in each orbital, it is .Therefore the N electron configuration will be 1s 2 2s 2 2p 3. Video: Nitrogen .
Chromium Since 1s can only hold two electrons the next 2 electrons for magnesium go in .We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since .
Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .
Lithium is the third element with a total of 3 electrons. In writing the electron .
How to Write the Electron Configuration for Boron. Boron is the fifth element with a .Electronic configuration of Cr 3 +. Cr is an exception where the last electron enters into the 3d orbital instead of 4s orbital to attain half-filled stability. The electronic .
Mar 23, 2023 To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number of .
Chromium is known for its unique electron configuration, which deviates from the standard rules due to its half-filled and fully filled subshells. Let’s dive deeper into the details of .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .
Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. First, recall that the n = 3 shell is the .By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer .What is the electron configuration of Cr 3+? An element’s electronic configuration is a representation of its atoms’ electrons symbolically that are arranged across different atomic orbitals. In simple words, the electron configuration is the arrangement of electrons in distinct orbits and orbitals of an atom in a specific order.RELATED QUESTIONS. Electronic configuration of species M 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 and its atomic weight is 56. The number of neutrons in the nucleus of species M is. Assertion: The spectrum of He + is expected to be similar to that of hydrogen. Reason: He + is also one electron system. The electronic configuration of Eu (Atomic no. 63) . To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number.Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written (here is an explanation why). Therefore we have (still incorrect) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9 4s 2. Correct Electron Configuration for Copper (Cu)
Write down the electronic configuration of:(i) Cr^3+(iii) Cu^+(v) Co^2+(vii) Mn^2+(ii) Pm^3+(iv) Ce^4+(vi) Lu^2+(viii) Th^4+📲PW App Link - https://bit.ly/PW.
The electronic configuration of C r (24) atom is: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 which is half-filled d-orbital. C r 3 + has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [A r] 3 d 3.
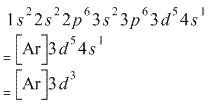
Writing out the electron configuration tells us how the electrons in an atom or ion are arranged in their shells, subshells and orbitals; This can be done using the full electron configuration or the shorthand version. The full electron configuration describes the arrangement of all electrons from the 1s subshell up; The shorthand electron .
The electron configuration of Cr3+ is [Ar] 3d3. This is because when a neutral chromium atom (Cr) gains three electrons, it will become a Cr3+ ion. By losing the three electrons, Cr is left with 17 remaining electrons, which is the same number of electrons as the Argon (Ar) element in the Periodic Table. .
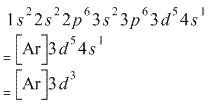
Write electronic configuration for C u, C u 2 +, Z n 2 +, C r 3 +. Open in App. Solution. Verified by Toppr. Electronic configuration for: 29 C u .electronic configuration of cr3 Chromium Write electronic configuration for C u, C u 2 +, Z n 2 +, C r 3 +. Open in App. Solution. Verified by Toppr. Electronic configuration for: 29 C u .
Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.When their electron configurations are added to the table (Figure 6.29), we also see a periodic recurrence of similar electron configurations in the outer shells of these elements. Because they are in the outer shells of an atom, valence electrons play the most important role in chemical reactions. The outer electrons have the highest energy of .
Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from .The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a .
Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written (here is an explanation why). Therefore we have 1s 2 2s 2 2p 6 3s 2 3p 6 3d .Click here:point_up_2:to get an answer to your question :writing_hand:the number of unpaired electrons in cr3 ion is Electronic configurations of `Cr^(3+)` (containing 21 electrons) is same as that of `Sc (Z = 21)`, i.e., isoelectronic species have the same electronic configuration Reason. Orbitals of atoms as well as ions are filled in order of increasing energy following aufbau principle A. If both assertion and reason are true, and reason is the true .
electronic configuration of cr3|Chromium
PH0 · What is the electron configuration of Cr 3+? Chemistry Q&A
PH1 · What is the electron configuration of Cr 3+?
PH2 · What is the electron configuration of Cr
PH3 · The electronic configuration of { Cr }^{ 3
PH4 · Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules)
PH5 · Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
PH6 · Electron Configuration for Chromium (Cr and Cr2+, Cr3+ ions)
PH7 · Electron Configuration For Chromium
PH8 · Electron Configuration Chart of All Elements (Full Chart)
PH9 · Chromium (Cr)
PH10 · Chromium
PH11 · 2.6: Electron Configurations
PH12 · 1.9: Electron Configurations for Transition Metal Elements